|
||
| Sulphuric Acid on the WebTM | Technical Manual | DKL Engineering, Inc. |
Knowledge for the
Sulphuric Acid Industry
![]()
Sulphuric Acid on the Web
Introduction
General
Equipment Suppliers
Contractor
Instrumentation
Industry News
Maintenance
Acid
Traders
Organizations
Fabricators
Conferences
Used
Plants
Intellectual
Propoerty
Acid
Plant Database
Market
Information
Library
Technical Manual
Introduction
General
Definitions
Instrumentation
Plant Safety
Metallurgial
Processes
Metallurgical
Sulphur Burning
Acid Regeneration
Lead Chamber
Technology
Gas Cleaning
Contact
Strong Acid
Acid Storage
Loading/Unloading
Transportation
Sulphur
Systems
Liquid SO2
Boiler Feed Water
Steam Systems
Cooling Water
Effluent Treatment
Utilities
Construction
Maintenance
Inspection
Analytical Procedures
Materials of Construction
Corrosion
Properties
Vendor Data
DKL Engineering, Inc.
Handbook of Sulphuric Acid Manufacturing
Order
Form
Preface
Contents
Feedback
Sulphuric Acid
Decolourization
Order Form
Preface
Table of Contents
Process Engineering Data Sheets - PEDS
Order
Form
Table of Contents
Introduction
Bibliography of Sulphuric Acid Technology
Order Form
Preface
Contents
Properties - Sulphuric Acid
September 27, 2003
| Introduction | Associated Links |
Introduction
Sulphuric acid is a colourless to amber, slightly cloudy, oily liquid with a specific gravity almost twice that of water. At normal temperatures and a concentration of 98% or lower, it has little odour. At higher temperatures and above a concentration of 98% irritating sulphur trioxide (SO3) fumes may be liberated. Oleum (fuming sulphuric acid) is a colourless to white, heavy, oily liquid containing sulphur trioxide dissolved in sulphuric acid. On exposure to air, oleum releases irritating white fumes of sulphur trioxide.
All grades of sulphuric acid have an affinity for water resulting in evolution of heat when diluted. In the case of oleum, the reaction occurs with explosive violence. Sulphuric acid and oleum will absorb moisture from the air resulting in dilution of the acid. All grades are strongly corrosive and personal safety precautions should be carefully followed.
Many organic substances, wood, starch, sugar, paper, etc. are charred on contact with sulfuric acid, forming carbon. Very small quantities of organic matter will blacken sulphuric acid as a result of suspended carbon.
| Colour | Clear, colourless liquid Contaminants in the acid may impart a colour to the acid. |
| Reactivity | Sulphuric acid will attack many metals and in its concentrated form is a strong oxidizing agent and may cause ignition on contact with organic materials, nitrates, carbides, chlorates, etc. |
| Flash Point | None |
| Structure | 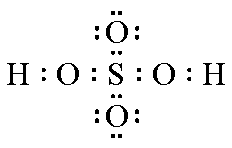 |
| Specific Gravity | Varies with concentration and temperature. See Table See Chart |
| Freezing Point | See Table See Chart |
| Boiling Point | See Table See Chart |
| Viscosity | See Chart |
| Conductivity | See Chart |
| Enthalpy | See Chart |